 United States Current Temperatures
United States Current TemperaturesCurrent temperatures color contoured every 5 degrees F.
The Current Temperature map shows the current temperatures color contoured every 5 degrees F. Temperature is a physical property of a system that underlies the common notions of hot and cold; something that is hotter generally has the greater temperature. Specifically, temperature is a property of matter. Temperature is one of the principal parameters of thermodynamics. On the microscopic scale, temperature is defined as the average energy of microscopic motions of a single particle in the system per degree of freedom. On the macroscopic scale, temperature is the unique physical property that determines the direction of heat flow between two objects placed in thermal contact. If no heat flow occurs, the two objects have the same temperature; otherwise heat flows from the hotter object to the colder object. These two basic principles are stated in the zeroth law and second law of thermodynamics, respectively. For a solid, these microscopic motions are principally the vibrations of its atoms about their sites in the solid. For an ideal monatomic gas, the microscopic motions are the translational motions of the constituent gas particles. For a multiatomic gas, vibrational and rotational motion should be included too.
Temperature is measured with thermometers that may be calibrated to a variety of temperature scales. In most of the world (except for the United States, Jamaica, and a few other countries), the degree Celsius scale is used for most temperature measuring purposes. The entire scientific world (the U.S. included) measures temperature using the Celsius scale and thermodynamic temperature using the kelvin scale, which is just the Celsius scale shifted downwards so that 0 K= −273.15°C, or absolute zero. Many engineering fields in the U.S., especially high-tech ones, also use the kelvin and degrees Celsius scales. However, the United States is the last major country in which the degree Fahrenheit temperature scale is used by most lay people, industry, popular meteorology, and government. Other engineering fields in the U.S. also rely upon the Rankine scale (a shifted Fahrenheit scale) when working in thermodynamic-related disciplines such as combustion.
Intuitively, temperature is a measure of how hot or cold something is, although the most immediate way in which we can measure this, by feeling it, is unreliable, resulting in the phenomenon of felt air temperature, which can differ at varying degrees from actual temperature. On the molecular level, temperature is the result of the motion of particles which make up a substance. Temperature increases as the energy of this motion increases. The motion may be the translational motion of the particle, or the internal energy of the particle due to molecular vibration or the excitation of an electron energy level. Although very specialized laboratory equipment is required to directly detect the translational thermal motions, thermal collisions by atoms or molecules with small particles suspended in a fluid produces Brownian motion that can be seen with an ordinary microscope. The thermal motions of atoms are very fast and temperatures close to absolute zero are required to directly observe them. For instance, when scientists at the NIST achieved a record-setting cold temperature of 700 nK (1 nK = 10−9 K) in 1994, they used optical lattice laser equipment to adiabatically cool caesium atoms. They then turned off the entrapment lasers and directly measured atom velocities of 7 mm per second in order to calculate their temperature.
 United States Current Temperatures
United States Current TemperaturesCurrent temperatures color contoured every 5 degrees F.
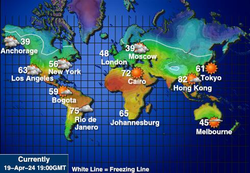 Global Temperatures
Global TemperaturesLines of equal temperature in degrees Celsius.
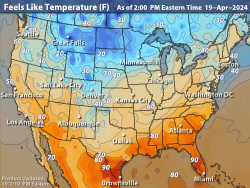 Feels Like Temps
Feels Like TempsThe Feels Like Temperatures map show what the outdoor temperature will feel like for the current day. Feels Like Index is a factored mixture of the Wind Chill Factor and the Heat Index.
 Frost & Freeze Today
Frost & Freeze TodayThe Frost and Freeze map shows where frost cant be expected and where temperature are forecast to fall below 32 degrees F.
 Frost & Freeze Tomorrow
Frost & Freeze TomorrowThe Frost and Freeze map shows where frost cant be expected and where temperature are forecast to fall below 32 degrees F.